The Cohen Lab develops and applies new tools to study biology. We push the limits of physics and chemistry to make measurements in previously inaccessible regimes. Work in the lab combines optics, protein engineering, chemistry, electrophysiology, simulation, and theory. We work at the levels of individual molecules, single cells, and whole, genetically modified, organisms.
A major focus is toward studying electrical signaling in the nervous system. We developed fluorescent reporters of membrane voltage and associated instrumentation to map electrical activity with high resolution in space and time. We study information processing in the brains of awake, behaving mice and zebrafish. We also work on electrical signaling in other tissues; on mechanical signaling in cells; and on the roles of physical forces in embryonic development.
Research Areas
News
Looking for postdocs
We're looking for postdocs to join our lab! Possible projects include:
- Develop tickertape-like protein assemblies for brain-wide neural recording.
- Study dendritic integration, back-propagation, and plasticity.
- Perform ultra-wide-aread all-optical electrophysiology experiments to study neural dynamics in vivo.
- Develop software, optics, and analysis methods to support any of the above!
If interested, please email cover letter, CV and names of references to Adam Cohen.
Nothing fishy about this tale (11/2025)
Bill Jia and Xin Tang showed that when a zebrafish twitches its tail, the vascular endothelial cells respond with a large calcium transient. This response is mediated by Piezo1, and can be triggered just by pushing on the fish. Vascular mechanosensation is more about sensing body motion than basal blood flow (at least in this case)! Now out in Current Biology.
Following current events (7/2025)
Maddie Howell used diffusional confinement in cell-attached membrane tethers to localize the calcium transported by opening of single voltage-gated calcium channels. This is more sensitive than single-channel patch clamp, and could be generalized to record activity of non-electrogenic transporters or enzymes (when paired with a suitable fluorescent reporter). Out now in ACS Nano. Totally tubular work, Maddie!
Far-red calcium indicator (5/2025)
Robert Campbell's lab developed a far-red calcium indicator, excitable at 635 nm. We helped characterize it. Published in Nature Communications. We've paired FR-GECO1c with blue light optogenetic stimulation in studies on zebrafish hearts and on calcium transport in dendrites. These reporters work really well!
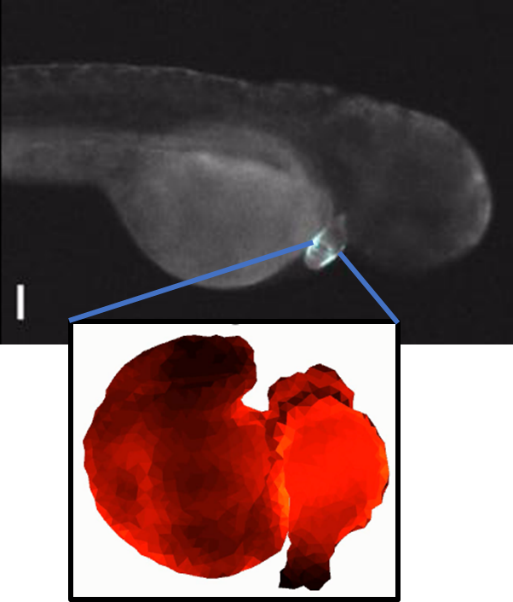
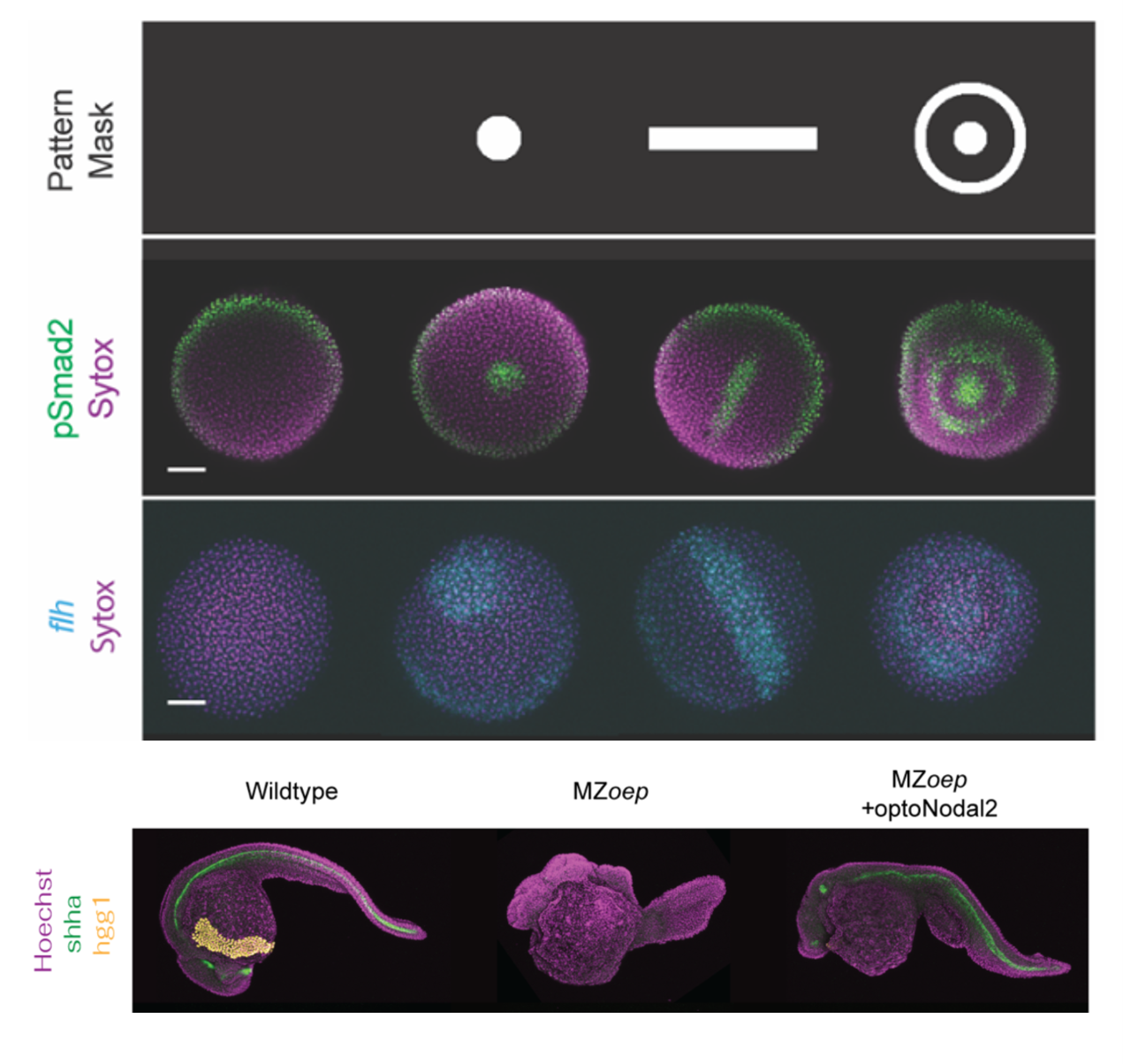
Sculpting embryonic development with light (4/2025)
Nate Lord and Harry McNamara led an effort to develop "OptoNodal2", a light-gated mimic of the Nodal signaling pathway. They can sculpt zebrafish development with light! Published in Development. Great collaboration with Alex Schier's lab.
What is the shape of a memory? (3/2025)
Doyeon developed a pulse-chase labeling tool to tag freshly potentiated synapses in vivo, published in Nature Neuroscience. There's a surprisingly tight correlation between synaptic plasticity and cFos expression. Nice work, Doyeon!
See also a nice news story about this work in the Harvard Gazette.
What is the role of dendritic excitations? (2/2025)
Pojeong Park and David Wong-Campos teamed up to make detailed maps of dendritic excitations in hippocampal pyramidal cells, published in Nature Communications. In acute slices, dendritic excitations seem primarily to mediate spike back-propagation, rather than dendritic integration. Check out the beautiful movies! See also a summary of this work in the Harvard Brain Science Initiative newsletter.
Photochemical labeling in vivo (1/2025)
Working with our colleagues in Luping Liu's lab, Sarah Innes-Gold used photocaged tetrazines to print stripes and other patterns on cells and fish. She made a toolbox of genetically and chemically targeted photocaged tetrazines. Published in ACS Chemical Biology. Patterned photochemistry is such a powerful approach.
Opsin photophysics rise again (1/2025)
In FRET-opsin voltage indicators, 2P excitation typically gives bright fluorescence, but no voltage sensitivity. Phil, Daozheng, and Hunter discovered that in these proteins the voltage-sensitive states are a photocycle intermediate. 2P excitation doesn't typically populate these states, but suitably engineered scan profiles can restore voltage sensitivity, allowing 2P voltage imaging in vivo with Voltron2. Published in Science Advances. The wonders of opsin photophysics never cease!
Calcium transport in dendrites (11/2024)
How far and how fast does calcium move through a dendrite? Rebecca combined all-optical perturbation and mapping of calcium dynamics to probe calcium flows and homeostasis in dendrites. Published in Cell Calcium. Another demonstration of the power of all-optical physiology techniques.
Uterine contractions (10/2024)
What is the structure of uterine contractions? David Combs mapped calcium dynamics throughout the estrus cycle in non-pregnant mice. Published in PNAS Nexus. Many thanks to Sarah England at Wash. U. for being our guide to the uterus.
2P or not 2P (redux) (7/2024)
Two-photon imaging provides great depth penetration in tissue, but not a lot of photons. In this Neurophotonics paper, Hunter and Phil analyzed the shot-noise limits on 2P voltage imaging. It's really hard to get enough photons without optically damaging the tissue.
Excitations on the edge (1/2024)
Interfaces between two non-excitable systems can be excitable. We showed this happens in electrophysiology, ecological models, and chemical reaction-diffusion networks, and these interfacial excitations can be remarkably robust. Out now in PNAS. Great collaboration with Colin Scheibner and Vincenzo Vitelli at U. Chicago. Exciting work!
In matters of the heart, is the first time special? (9/2023)
Bill's paper on the first beats of a zebrafish heart is out in Nature. He watched the heart go from not-beating to beating, and he made a dynamical model of how this transition happens. Great collaboration with Sean Megason. Nice work, Bill!
Voltage imaging, in a flash (9/2023)
A collaborative paper with Young-Gyu Yoon's group in Nature Methods describes a new machine learning-based approach to signal extraction from voltage imaging data. Data from Pojeong Park and Urs Boehm. Happy to SUPPORT this effort!
A sour note on optogenetics (8/2023)
Rebecca showed that chronic channelrhodopsin stimulation acidifies cells via opsin-mediated proton conductance. This effect is bigger in small structures like dendrites than in bigger structures like somas. Then she characterized some proton-impermeable opsins which don't have this problem! Read about it in eLife.
Welcome to Xiang Wu (7/2023)
Xiang is starting his postdoc in the lab after finishing his PhD in Guosong Hong's lab at Stanford. Xiang will be developing high-speed volumetric voltage imaging systems. Welcome, Xiang!
Sackler Prize in Chemistry (7/2023)
Adam went to Israel to accept the Sackler Prize in Chemistry. It was great to see old friends and make many new ones!
All-optical cAMP-ology (6/2023)
Katherine developed tools and techniques for simultaneous optical perturbation and optical measurement of cAMP. She studied how cAMP diffuses down dendrites and she made elegant scaling calculations of the relation betwen cAMP dynamics and neurite geometry. Beautifully done, Katherine!
A trio of papers on neuronal biophysics and plasticity (5/2023)
We posted three preprints on new techniques and applications for mapping single-neuron dynamics associated with plasticity. This effort represents our lab's first forray into the connection between biophysics and information processing. So much more to do here!
- Mapping memories: Doyeon Kim developed a pulse-chase technique to label freshly surface-exposed AMPA receptors in vivo. He found a surprisingly strong correlation between AMPAR exocytosis and cFos expression in mice that had undergone a contextual fear conditioning protocol. Here's the preprint.
- From dendritic biophysics to learning rules: Pojeong Park led an effort to map voltage propagation in CA1 pyramidal cells in acute slices. By combining voltage imaging, optogenetics, and pharmacology, he determined that dendritic nonlinearities played a bigger role in back-propagation than in dendritic integration. Pojeong discovered an intuitive explanation for how dendritic ion channel biophysics govern plasticity and metaplasticity rules. Here's the preprint.
- Dendritic voltage imaging in vivo: David Wong-Campos led an effort to map dendritic voltages in vivo, in L2/3 pyramidal cells. He found that voltages were highly correlated across the dendritic tree, but that dendrites acted as a spike-rate accelerometer on somatically generated action potentials. He found little evidence for locally generated dendritic spikes. Here's the preprint.
Time-tagged ticker tapes (5/2023)
Dingchang and Ted developed protein fibrils which could record a history of gene expression in a single cell! Check out their cover story in Nature Biotechnology. Great collaboration with groups of Luke Lavis and David Baker.
Hippocampal plasticity in vivo (1/2023)
Linlin used Tian's new QuasAr6a voltage indicator to make long-term hippocampal recordings in a mouse navigating virtual reality. She optogenetically evoked place fields, and mapped the changes in synaptic strength. Out now in Cell. Tour de force from Linlin and great collaboration with the Deisseroth Lab.
New far-red voltage indicators (1/2023)
Tian engineered a new far-red Arch-based voltage indicator, QuasAr6a, out in Nature Methods. As impressive as the reporter is how she did it: using a video-based pooled screen from a single-pot library of mutants. Spiking HEK cells for the win!
Topological electrophysiology (1/2023)
Hillel discovered topological action potentials, the most twisted electrophysiology you ever saw. Now out in Nature Physics. The interface between two non-excitable tissues can be excitable--more robustly so than any bulk excitable tissue. Implications for arrhythmias and development? Nice work, Hillel!
Stretching our minds, or at least our neurons (8/2022)
Zheng and Sarah discovered that membrane tension propagates pretty quickly (~20 microns/s) in growing axons, now out in Science Advances. They showed that tension coordinated long-range competition between extension of existing growth cones vs nucleating new branches closer to the soma. Is this a mechanism for mechanical obstacle avoidance in axon guidance?
2P or not 2P (6/2022)
That is the question. Hunter Davis won a Best Poster Prize at the recent conference on Sculpted Light in the Brain. Congratulations, Hunter! His poster compared one-photon and two-photon approaches to voltage imaging.
Twinkle, twinkle, little astrocyte! (4/2022)
Moritz Ambruster and Chris Dulla did voltage imaging in astrocytes, now out in Nature Neuroscience. They observed surprisingly localized fluctuations in voltage, modulated by nearby neural activity. Yoav and I helped out on the voltage imaging and interpretation. Congratulations to Moritz, Chris and team!
Lighting up dendrites (3/2022)
Andrew Landau and Bernardo Sabatini did a beautiful study of back-propagating action potentials in L2/3 pyramidal cells, now out in eLife. We chipped in some voltage imaging using Tian's new QuasAr6 voltage indicator, paired with calcium imaging. Congratulations to Pojeong Park (electrophysiology), David Wong-Campos (instrumentation), and Tian He (protein engineering). Great to collaborate with Bernardo and team!
Tales of fish tails (1/2022)
Urs' major paper just came out in Neuron! He combined light-sheet voltage imaging, optogenetic stimulation, and genetic knockout to characterize excitatory spinal neurons in zebrafish swimming in a virtual reality environment. He discovered that a previously uncharacterized population, V3, controls the strength of the swimming tail motions. Read Urs' tale in Neuron.
Polarized views of dendrites (11/2021)
Super (former) undergrad William Bloxham and postdoc Daan Brinks showed that the fluorescence of many protein and dye-based voltage indicators depends on the polarization of the incident light. By aligning the polarization with the transition dipole, you can substantially increase the brightness and signal-to-noise ratio! Read about it in Biophys. J.
Stimulating thoughts on emission (10/2021)
David Wong-Campos and Trey Porto analyzed the pattern of photon emission from a single excited fluorophore undergoing stimulated emission. It turns out to be super subtle and interesting. This paper was for a special issue of J. Phys. Chem., in honor of Adam's thesis advisor, W. E. Moerner. Our findings support the Moerner Lab ethos (with apologies to PW Anderson), Fewer is Different!
Fellowships and prizes galore! (7/2021)
So many awards for the great members of the team:
- Phil Brooks received a graduate Harvey Fellows Award (4/2020)
- Hillel Ori received an EMBO Postdoctoral Fellowship (7/2020)
- Michael Xie got a Hoopes prize for his undergraduate thesis (5/2021)
- David Combs received a Young Investigator Grant from the Society for Obstetric Anesthesia and Perinatology (SOAP) (5/2021)
- Sarah Innes-Gold received a Jane Coffin Childs Postdoctoral Fellowship (5/2021).
- Bill Jia received the John S. LaDue Graduate Fellowship in cardiovascular medicine and a NSF-Simons QBio Graduate Fellowship (6/2021)
- Dingchang Lin received a Harvard Brain Initiative Young Scientist Transition Award (6/2021)
- Tian He received a Michael Davidson and Roger Tsien Commemorative Conference Award from Addgene (7/2021)
- David Wong-Campos received a Life Sciences Research Foundation Postdoctoral Fellowship (7/2021)
Light up neurons like a super NovA(rch) (5/2021)
Miao and Daan developed Photoactivated voltage imaging in tissue with an archaerhodopsin-derived reporter, out in Science Advances. When tickled with blue or 2-photon light, the far-red fluorescent reporter gets up to seven-fold brighter. It's called NovArch (get it?). We can now map voltage in dendrites in tissue!
Get the voltage imaging wiggles (4/2021)
Super-undergrad Michael Xie led a multi-lab effort to develop a data analysis pipeline for High-fidelity estimates of spikes and subthreshold waveforms from 1-photon voltage imaging in vivo, out in Cell Reports. Great collaboration with labs of Liam Paninski and Karel Svoboda! This pipeline distinguishes real subthreshold events from out-of-focus crosstalk and other optical artifacts.
Welcome Maddie! (4/2021)
Madeleine Howell officially joins the lab! Maddie will be working on optomechanical imaging systems to probe microscale mechanical properties of cells and tissues. Welcome, aboard, Maddie!
MOSAIC: Multiplexed optical sensors in arrayed islands of cells (8/2020)
Kit developed a technique to record from > 20 fluorescent reporters simultaneously, monitoring how many different metabolites and signaling molecules respond to physiological perturbations. Powerful new tool, out in Nature Communications. Great collaboration with Emil Hansson!
![]()
Lateral inhibition in cortical L1 (1/2020)
Linlin used Optopatch and an inhibitory version, i-Optopatch, to dissect the role of lateral inhibition in sensory processing cortical Layer 1. Check out the paper in Cell. Great collaboration with Anne Takesian and Ed Boyden labs.
Bioelectrical domain walls (1/2020)
Harry discovered that homogeneous tissues can spontaneously break spatial symmetry to form bioelectrical domain walls. Check out the paper in Nature Physics. Great collaboration with Olivier Pourquie and lab.
Jiggling like Jello (11/2019)
Zheng and Adam wrote a review about cell membrane mechanics Do cell membranes flow like honey or jiggle like jello? Here's some food for thought, just out in BioEssays. See also the video abstract:
All-optical electrophysiology in behaving mice (4/2019)
Yoav's epic project to develop optical electrophysiology in awake, behaving mice just came out in Nature! Voltage imaging of spikes and subthreshold events reveal interesting hippocampal dynamics associated with locomotion.
All-optical neurophysiology in brain slices (4/2019)
Sami and Vicente combined Hadamard microscopy with a new blue-shifted channelrhodopsin and a red-shifted, nuclear-localized Ca++ indicator for ultrawide-field all-optical neurophysiology in brain slices. Find out how your favorite drug acts, brain wide! Published in Journal of Neuroscience. Cover Story!
High-speed Hadamard Microscopy (1/2019)
Vicente and Carlos developed a beautiful imaging technique that combines structured illumination with Hadamard sequences and compressed sensing. See their paper on Compressed Hadamard Imaging in J. Phys. D. Using one-photon illumination, they achieved high-speed optically sectioned imaging in tissue. Come for the math, stay for the images!
Cells and gels in Cell (11/2018)
Zheng's paper on cell membrane mechanics just came out in Cell. He showed that tension in the cell membrane is highly localized, and propagates slowly and diffusively. This is counter to the widely held view of the cell membrane as a freely flowing liquid: think Jello, not honey!
Artificial arrhythmias (10/2018)
Harry's paper on the geometry-dependent transition between regular spiking and arrhythmia in spiking HEK cells was the cover story in Cell Systems. He showed that even in a very simple excitable cell type, the spiking dynamics are sensitive to the overall tissue geometry through long-range electrotonic coupling. This is a challenge for using human stem cell-derived cardiomyocytes for disease modeling or cardiotoxicity screening. Be still, my beating heart!
synOptopatch in Nature Methods (10/2018)
Linlin's paper on an all-optical assay for synaptic transmission just came out in Nature Methods! She also showed that ketamine induces disinhibition by primarily blocking excitatory-to-inhibitory synapses. Bravo, Linlin!
Poster children (7/2018)
Two labmembers won 'Best Poster' awards at separate conferences this summer. Congratulations to Zheng for winning at the FASEB conference on Molecular Biophysics of Membranes. Congratulations to Yoav for winning at the Gordon Research Conference on Optogenetics and Imaging. Hooray!
Cell membranes resist flow (5/2018)
Zheng's paper is on the bioRxiv! Cell membranes are more like Jello than maple syrup. This was a surprise to us--maybe to you too!
All-optical electrophysiology in awake, behaving, mice (3/2018)
Yoav's paper is on the bioRxiv! This heroic piece of work combines new voltage indicators, new microscopes, new mice, and new software. Great team effort! All to see how the hippocampus represents locomotion.
Archive
Contact:
tel: (617) 496-9466
email: cohen@chemistry.harvard.edu
Office: Mallinckrodt 115
Harvard University
12 Oxford Street
Cambridge, MA 02138
Lab Manager: Hillary Piccerillo
tel: (617) 496-8233
email: hpiccerillo@fas.harvard.edu